- Atomic Configuration and Bonding Characteristics
(1) Layered Crystal System
Graphite exhibits a unique 2D honeycomb lattice structure. Each carbon atom forms covalent bonds (1.42Å bond length, 120° bond angle) with three adjacent atoms through sp² hybridized orbitals. This planar structure creates atomically thin layers (0.335nm thickness) held together by van der Waals forces (~2eV/atom), with interlayer bonding strength only 1/300 of intra-layer covalent bonds.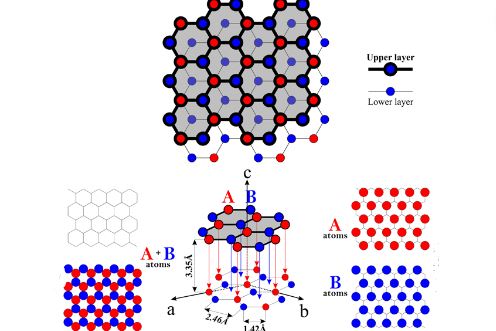
(2) Electron Conduction Mechanism
The fourth p-orbital electron from each carbon atom forms delocalized π bonds, creating a continuous electron cloud within the plane. This 2D electron gas system enables anisotropic electrical conductivity – in-plane conductivity reaches 3×10⁶ S/m (surpassing lead metal), while cross-plane conductivity drops by three orders of magnitude.
- Physical Performance Parameters
(1) Mechanical Properties
Interlayer shear modulus: 0.5GPa | Mohs hardness: 1.5 | Monolayer tensile strength: 130GPa (theoretical). This mechanical anisotropy combines lubricity with structural strength (friction coefficient 0.1), making it ideal for high-load bearings.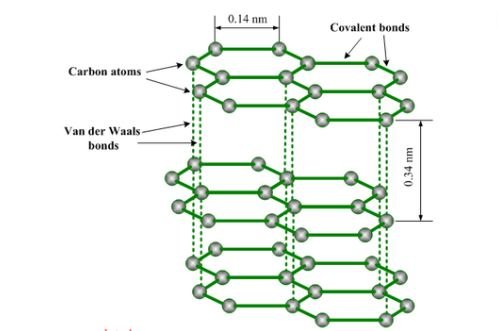
(2) Thermodynamic Characteristics
In-plane thermal conductivity: 1500-2000 W/(m·K) (exceeds copper) | Cross-plane: 5-10 W/(m·K). Sublimation temperature: 3650℃ | Negative thermal expansion anisotropy (in-plane: -1×10⁻⁶/K; cross-plane: 29×10⁻⁶/K).
III. Chemical Stability Analysis
(1) Oxidation Resistance
Stable in most acids/alkalis (except concentrated HNO₃) | Begins slow oxidation with O₂ at 400℃ (activation energy ~190kJ/mol) | Weight loss rate <0.1%/year in 10⁻⁶ Torr vacuum.
(2) Intercalation Reactions
Forms graphite intercalation compounds (GICs), e.g., H₂SO₄-intercalated product with conductivity up to 1.5×10⁷ S/m. Critical material for Li-ion battery anodes (theoretical capacity: 372mAh/g).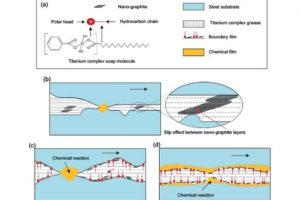
- Industrial Application Topology
(1) Functional Materials
• Nuclear reactor neutron moderators (absorption cross-section 0.0035 barns)
• Conductive fillers (percolation threshold 0.5vol%)
• High-temperature molds (thermal shock resistance >100 cycles)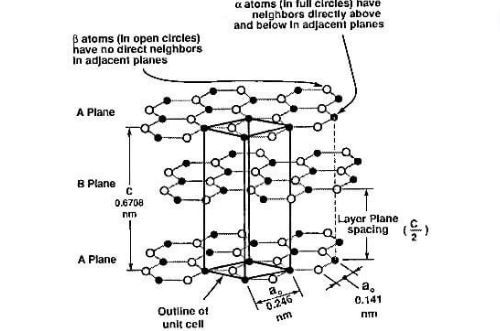
(2) Emerging Technologies
• Graphene precursor (mechanical exfoliation yield >90%)
• Flexible electronics substrate (surface roughness <0.5nm)
• Superconducting composite carrier (MgB₂ critical temperature 39K)
- Key Parameter Comparison
| Property | Graphite | Diamond | Ratio |
| Density (g/cm³) | 2.09-2.23 | 3.515 | 0.63 |
| Thermal Conduct. | 1950(∥) | 2200 | 0.89 |
| Carrier Density | 10¹⁸ cm⁻³ | <10¹⁰ cm⁻³ | 10⁸× |
This material system’s multiscale characteristics stem from its unique electronic structure and dimensional confinement effects, maintaining irreplaceable value in modern industry. Understanding its anisotropic nature is crucial for developing novel graphite-based composites.